General principles and limitations in field evaluations
Screening tests
The erythrocyte protoporphyrin level is not sensitive enough to identify children with elevated PbB levels below about 25 mcg/dl. The screening test of choice is now PbB measurement (CDC 1991).
Dose-response curve
When assessing the public health impact of environmental lead contamination, the lower portion of the dose-response curve for PbB vs. soil lead should be used. This portion of the curve has the steepest slope, and it corresponds to conditions in which the impact on PbB is the greatest.
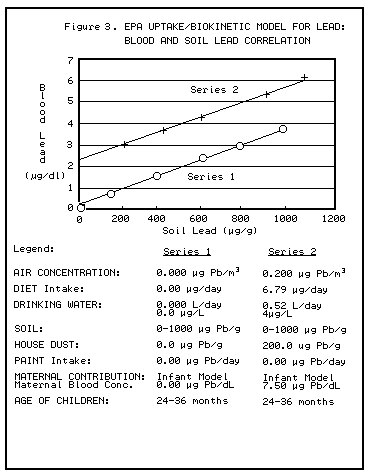
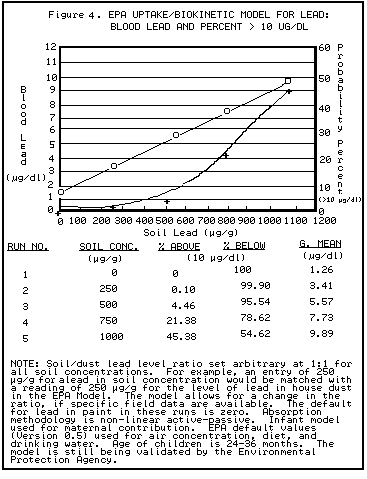
PbB levels generally rise 3-7 mcg/dl for every 1,000-ppm increase in soil or dust lead concentrations (CDC 1991). Access to soil, behavior patterns, presence of ground cover, seasonal variation of exposure conditions, and other factors may influence this relationship.
Sample size
Caution should be used in drawing conclusions when only one or a few soil samples from a site have been analyzed. Depending on the uniformity of lead distribution at a site, a single soil sample may significantly overestimate or underestimate the average lead concentration at a site.
Surface soil
Because lead is immobilized by the organic component of soil, lead deposited from the air is generally retained in the upper 2-5 centimeters of undisturbed soil (EPA 1986). Urban soils and other soils that are disturbed or turned under may be contaminated down to far greater depths. Opportunity for exposure is much greater to surface soil than to subsurface soils.
Evidence for the non-uniformity of lead distribution in urban soils was demonstrated in a study that examined soil lead concentrations in urban Baltimore gardens (Chaney 1984). Soil lead concentrations varied more than 10-fold within a single garden.
Chemical form of lead
The impact of exposure to lead-contaminated soil on PbB levels is also influenced by the chemical and physical form of the lead. Data from animal feeding studies suggest that the oral bioavailability of lead sulfide and lead chromate is significantly less than the bioavailability of other lead salts (oxide, acetate) (Barltrop and Meek 1975).
Particle size
Increasing the particulate size also reduces the bioavailability of lead in the gastrointestinal tract. In animal feeding studies, decreasing the lead particulate size from 197 microns to 6 microns resulted in a 5-fold enhancement in absorption (Barltrop and Meek 1979). The lead content of soil and dust has also been demonstrated to vary dramatically as a function of particle size (Duggan and Inskip, 1985). Several studies have reported that the lead content of soil, street dust, city dust, and house dust increases as the particle size decreases.
Lead-mining sites
The results of studies at lead-mining sites have indicated that soil lead contamination from mine tailings may be less effective in increasing PbB levels than is lead contamination derived from urban lead pollution (paint, gasoline) or atmospheric lead fallout from lead smelting operations (Steele et al. 1990). However, an animal study by LaVelle et al. (1991) on the bioavailability of lead in mining wastes following oral intubation in young swine does not support these findings.
The reduced bioavailability of lead from mine tailings may be related to its chemical form (lead sulfide) and its larger particulate size. Evaluations of mining sites require analyses of these physical-chemical parameters.
Pathways of Exposure
Soil and dust act as pathways to children for lead deposited by primary lead
sources such as lead paint, leaded gasoline, and industrial or occupational
sources of lead (CDC 1991).
Because lead does not dissipate, biodegrade, or decay, the lead deposited
into dust and soil becomes a long-term source of lead exposure for children. For
example, although lead emissions from gasoline have largely been eliminated, an
estimated 4-5 million metric tons of lead previously used in gasoline remain in
dust and soil, and children continue to be exposed to it (ATSDR 1988).
Prevention activities
Community prevention activities should be triggered by PbB levels > or =
10 mcg/dl, as recommended by the Centers for Disease Control (Table 8), (CDC, 1991).
For community-level intervention to be successful at least five types of
activities are necessary (CDC, 1991).
(1) screening and surveillance (2) risk assessment and integrated prevention planning
(3) outreach and education (4) infrastructure development (5) hazard reduction Soil lead abatement
Exposure Pathways and Populations at Risk
Soil and dust act as pathways to children for lead deposited by primary lead
sources such as lead in paint, leaded gasoline, and industrial or occupational
sources of lead. Because lead does not dissipate, biodegrade, or decay, the lead
deposited into dust and soil becomes a long-term source of lead exposure for
children.
Preschool-age children and fetuses are usually the most vulnerable segments
of the population for exposure to lead. Among children, those in the 2-3
year-old age bracket may be most at risk for exposure to lead-contaminated soil.
The number of children potentially exposed to lead in dust and soil is estimated
at 5.9 to 11.7 million children.
Uptake and Bioavailability of Lead
A strong positive correlation is found between exposure to lead-
contaminated soils and PbB levels. Generally, the PbB levels rise 3-7 mcg/dl for
every 1000 ppm increase in soil or dust concentrations. Access to soil, behavior
patterns, presence of ground cover, seasonal variation of exposure conditions,
and other factors may influence this relationship.
Bioavailability of lead in the gastrointestinal tract is influenced and may
be reduced as the particulate size of lead is increased. The reduced
bioavailability of lead from mine tailings may be related to its chemical form
and its larger particulate size. Evaluations of mining sites require analyses of
these physical- chemical parameters.
Biomarkers
The most commonly used biomarkers of lead exposure are the PbB
concentration and the blood erythrocyte protoporphyrin (EP) concentration. The
EP test has poor sensitivity and specificity below a PbB level of 25 mcg/dl. The
CDC recommends PbB concentration as the screening test of choice.
Site-Specific Exposure Assessment
Interactive and complex factors associated with multiple exposure pathways
for lead require a site-specific approach in order to develop meaningful action
levels for lead in soil. When evaluating a site, a health assessor should be
aware of multiple sources of lead exposure and the additive nature of the risks.
Dust and soil lead -- derived from flaking, weathering, and chalking paint --
plus airborne lead fallout and waste disposal over the years, are the major
proximate sources of potential childhood lead exposure.
Wide variations in soil lead levels have been reported, ranging from less
than 100 ppm to well over 11,000 ppm. Soils adjacent to houses with exterior
lead-based paints may have lead levels of >10,000 mcg/g. The downward
movement of lead from soil by leaching is very slow under most natural
conditions.
At a site, the health assessor should examine the available PbB data.
Recently, the CDC has provided guidelines for interpreting PbB test results in
children. If conditions at a site change dramatically, retesting exposed
individuals may be necessary to determine the impact of altered conditions on
PbB levels. The health assessor should pay attention to potential seasonal
variation of exposure conditions; the half-life of lead in the blood stream; and
limitations of any screening methods used, especially study design (power and
representativeness of blood and soil samples), should be evaluated.
The health assessor should use caution in drawing conclusions when only one
or a few soil samples from a site have been analyzed. Depending on the
uniformity of lead distribution at a site, a single soil sample may
significantly overestimate or underestimate the average lead concentration at a
site. The impact of exposure to lead-contaminated soil on PbB levels is also
influenced by the chemical and physical form of the lead.
ATSDR Recommendations
At all sites, ATSDR recommends that health assessors evaluate the
need for any follow-up health activities. This effort should be coordinated with
other health agencies, as appropriate, to ensure that all aspects of a site that
impact the health of the community are evaluated. The recent statement by the
CDC, Preventing Lead Poisoning in Young Children, provides guidance and
identifies community prevention activities that should be triggered by PbB
levels > or = 10 mcg/dl.
American Academy of Pediatrics (AAP) (1987) (Committee on Environmental
Hazards and Committee on Accident and Poison Prevention of the American Academy
of Pediatrics). Statement on childhood lead poisoning. Pediatrics, 79,457-65.
Agency for Toxic Substances and Disease Registry (ATSDR) (1988). The nature
and extent of lead poisoning in children in the United States: A report to
Congress, July 1988.
Agency for Toxic Substances and disease Registry (ATSDR) (1990). Case studies
in environmental medicine: Lead toxicity.
Agency for Toxic Substances and Disease Registry (ATSDR) (1992).
Toxicological profile for lead, ATSDR/TP-88/17.
Barltrop D. and Meek F. (1975). Absorption of different lead compounds,
Postgrad Med J 51:805-9.
Barltrop D. and Meek F. (1979). Effect of particle size on lead absorption
from the gut. Arch Environ Health 34:280-5.
Binder, S. and Matte, T. (1992). Personal Communication. Review of soil lead
levels. May 8, 1992.
Centers for Disease Control (CDC), (1991). Preventing lead poisoning in young
children, October 1991.
Chaney RL, et al. (1984). The potential for heavy metal exposure from urban
gardens and soils, pages 37-84. In: J.R. Preer ed. Proceedings of the symposium
on heavy metals in urban gardens. Agricultural Experiment Station, University of
the District of Columbia, Washington.
Chisolm JJ, Jr. (1981). Dose effect relationship for lead in young children;
evidence in children for interactions among lead, zinc, and iron. (Cited in
ATSDR 1988)
Colorado Department of Health, (1990). University of Colorado at Denver,
Agency for Toxic Substances and Disease Registry, Leadville Metals Exposure
Study, April 1990.
Day JP, et al. (1979). Solubility and potential toxicity of lead in urban
street dust. Bull Environ Contam Toxicol 23:497-502.
Duggan MJ and Williams S. (1977). Lead in dust in city streets. Sci Total
Environ 7:91-7.
Duggan MJ. (1980). Lead in urban dust: an assessment. Water, Air, Soil
Pollution 14:309-21.
Duggan MJ and Inskip MJ. (1985). Childhood exposure to lead in surface dust
and soil: A community problem. Public Health Rev 13:1-54.
EPA (Environmental Protection Agency) (1986). Air quality criteria for lead,
June 1986 and Addendum, September 1986. Research Triangle Park, N.C., EPA
600/8-83-018F.
EPA (Environmental Protection Agency) (1990). Uptake/biokinetic model for
lead, Version 0.50 (December 1990).
EPA (Environmental Protection Agency) (1991). Three city urban soil-lead
demonstration project, midterm project update.
Hammond PB. (1982). Inorganic lead in man's environment: Sources and
toxicological significance. J Appl Toxicol 2(2):68-74.
Harrison RM. (1979). Toxic metals in street and household dusts. Sci Total
Environ 11:81-97.
Healy M, et al. (1982). Lead sulfide and traditional preparations: Routes for
ingestion and solubility and reactions in gastric fluid. J Clin Hosp Pharm
7:169-73.
Heard MJ and Chamberlain AC (1982). Effect of minerals and food on uptake of
lead from the gastrointestinal track in humans. Hum Toxicol 1:411-5.
LaVelle MJ, et al. (1991). Bioavailability of lead in mining wastes: an oral
intubation study in young swine (submitted for publication).
Madhaven S, et al. (1989). Lead in soil: Recommended maximum permissible
levels. Environ Res 49:136-42.
Mahaffey KR, et al. (1976). Difference in dietary intake of calcium,
phosphorus, and iron of children having normal and elevated blood lead
concentrations. J Nutr 106(7). (Cited in ATSDR 1988).
Mahaffey KR, et al. (1982). National estimates of blood lead levels: United
States, 1976-1980. N Engl J Med 307(10):573-9.
Markowitz ME and Rosen JF. (1981). Zinc (zn) and copper (Cu) metabolism in
CaNa2 EDTA-treated children with plumbism. Pediatr Res 15:635. (Cited in ATSDR
1988).
National Research Council (1980). Lead in the human environment. Washington,
DC: National Academy of Sciences.
Needleman HL, et al. (1990). The long-term effects of exposure to low doses
of lead in childhood: An 11-year follow-up report. N Engl J Med 322(2):83-8.
NSF (National Science Foundation) (1977). Lead in the environment.
NSF/RA-770214. Bogess,W.R., ed., NSF, Washington, D.C. (cited in EPA 1986a).
Que Hee SS, et al. (1985). Evolution of efficient methods to sample lead
sources, such as house dust and hand dust, in the homes of children. Environ Res
38:77-95.
Reagan PL and Silbergeld EK. (1989). Establishing a health based standard for
lead in residential soils. In: Hemphill and Cothern, eds. Trace substances in
environmental health, Supplement to Volume 12,(1990) of Environmental
Geochemistry and Health.
Schilling R and Bain RP. (1989). Prediction of children's blood lead levels
on the basis of household-specific soil lead levels. Am J Epidemiol
128(1):197-205.
Steele MJ, et al. (1990). Assessing the contribution from lead in mining
wastes to blood lead. Regul Toxicol Pharmacol 11:158-90.
Susten, A.S. (1990). The ATSDR health assessment: purpose, history, and
findings. In: JS Andrews, et al. (eds.), Environmental issues: Today's challenge
for the future. Proceedings of Fourth National Environmental Health Conference,
June 20-23, 1989, San Antonio, Texas. U.S. Department of Health and Human
Services, Public Health Service
Tola S, et al. (1973). Parameters indicative of absorption and biological
effects in new lead exposure: A prospective study. Br J Ind Med 30:134-41.
Watson WS, et al. (1980). Oral absorption of lead and iron. Lancet
(8188):236-7.
Yip R, et al. (1981). Iron status of children with elevated blood lead
concentrations. J Pediatr 98: 922-5.
To request a copy of this document or for questions concerning this document,
please contact the person or office listed below. If requesting a document,
please specify the complete name of the document as well as the address to which
you would like it mailed. Note that if a name is listed with the address below,
you may wish to contact this person via e-mail.
DR. CHARLIE XINTARASCommunity prevention activities
determining populations at
risk and the locations of the worst exposures;
analyzing all available data to assess sources of lead, exposure
patterns, and high-risk populations; developing prevention plans;
informing health-care providers,
parents, property owners, and other key people about lead poisoning prevention;
finding the resources needed
for a successful program of risk reduction;
reducing the hazards of lead-based
paint and lead in dust and soil, particularly in high-risk buildings and
neighborhoods.
Soil lead abatement may consist of either establishing an effective
barier between children and the soil or the removal and replacement of at
least the top few centimeters of soil. Summary
References
POINT OF CONTACT FOR THIS DOCUMENT:
Agency for Toxic Substances and Disease
Registry
1600 Clifton Road MS(E-28)
Atlanta, GA 30333
E-Mail:
chx1@cdc.gov
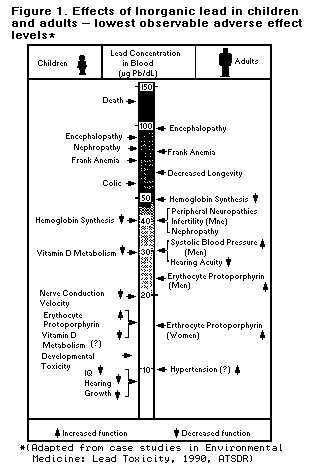
Effects Of Inorganic Lead On Children And Adults
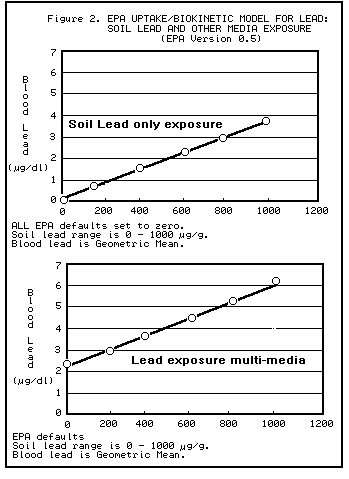
Soil Lead And Other Media Exposure
 Science
Corner
Science
Corner