The following article appeared in the Journal of Scientific Exploration, Volume 6, Pages 233-246, 1992.
The recent simulation of the miracle liquefying blood of Saint Januarius is shown to be viable from both historical and scientific standpoints. The history of the holy blood is traced and means and motivation for the simulation are provided. Spectral analysis of the simulated blood, a thixotropic gel of iron hydroxide (FeO(OH)), shows the absorption spectrum to be similar to old blood. Many reported characteristics of the holy blood can be explained by the behavior of a thixotropic gel. Modifications of the preparation procedure are attempted to bring the simulation into agreement with fourteenth century alchemical knowledge. A critical evaluation of previous spectroscopic studies of the miracle blood is presented.
Throughout the centuries, the study of miraculous events of a religious or cultist nature has proven to be far more elusive than the examination of paranormal events of the secular realm. This is, of course, the result of protection by the religious authority, which may feel either that the event needs no examination and should be accepted on faith, or that the investigation can only be performed by members of the religion or cult who already believe in the divine nature of the miracle. A truly "open-minded" evaluation of events is difficult to achieve under such conditions. The explanation for miraculous events ranges from hoax, hallucination, and coincidence to psychokinesis or divine action, depending on the viewpoint of the investigator. And similar to studies of paranormal events in the secular realm, a primary tool of the skeptical investigator has been duplication of the phenomena using materials or skills of a completely common nature. The validity of this approach depends on the ability of the investigator to prove means and motivation for the simulation. However, it is often necessitated by the limited access to the actual event given to the investigator and, short of subterfuge to obtain access, is usually the only way the study can be carried out. Cooperation between secular and religious authorities in the investigation of miracles, such as was realized in the study of the Shroud of Turin (Nickell, 1987), is the exception rather than the rule.
Miracles that can be conclusively studied by investigators using hard scientific disciplines, such as chemistry and physics, are limited to physical miracles of a nonmedical nature, that is, those miracles that are visible to more than one observer, are manifested as physical and paranormal phenomena, and do not involve healings. Examples include miraculous images, weeping icons, and unexplained transformations of physical state (i.e., coagulated blood that liquefies). The Roman Catholic Church has a long history of extensive investigation of miracles as a requirement for the beatification and canonization processes leading to sainthood (Woodward, 1990). However, the vast majority are physical miracles of a medical nature. In very few cases has the Roman Catholic Church accepted physical miracles of a nonmedical nature as evidence for sanctity. According to O'Collins and Farrugia (1991), "a miracle is an event which is caused by a special divine intervention, does not follow the normal laws of nature and carries a religious message for people now and later." Skepticism on the part of the Roman Catholic Church often results from the lack of a religious message associated with the phenomena. Other religious sects or cults are far more accepting of physical miracles and also far more restrictive towards investigations conducted by outsiders.
The recent simulation of a famous and venerated relic of the Roman Catholic Church, the holy blood of Saint Januarius (San Gennaro) is a perfect example of investigation by means of duplication using commonly available materials. The simulation was first described in a scientific communication to Nature (Garlaschelli, 1991). Supposedly collected in a vial after the beheading of the saint, the solid coagulated blood mysteriously liquefies during an elaborate ceremony that has been performed several times a year in Naples ever since the fourteenth century.
Based on very scant evidence, the story, almost a legend, of the Holy Blood begins with the historical Januarius, an early bishop of Benevento, who was arrested during the reign of the Emperor Diocletian in A.D. 305 for visiting and encouraging a deacon named Sosius. Januarius had previously seen flames around the head of Sosius while he was singing in church, a sign that was taken to mean that Sosius would soon wear the crown of martyrdom. It was a crown Januarius was to wear as well. Januarius, Sosius, and other clergy were exposed to wild beasts in the amphitheater, but when that didn't have the desired effect, the governor of Campania ordered them beheaded (Butler, 1955).
Januarius's relics were taken from near Pozzuoli to a catacomb located in Naples in the fifth century and he was declared the patron saint of the city. The saint's skull was apparently taken to the bishop's church. In 813, the bones were stolen by Sicone, a Lombard king during a war, and then taken to Benevento. The bones of the saint were hidden underground in the cloister of Montevergine (southern Italy) and were lost for several centuries, later to be found during works under the altar. The bones were taken to Naples by Frederick of Aragon. Charles II of Anjou had a silver reliquary built to contain the saint's head in 1304, and ceremonies honoring the saint were instituted by Archbishop Orsini of Naples in 1337, but nothing is mentioned about the blood until 1389, when it suddenly appeared in the diary of an anonymous Neapolitan: "On the seventeenth day (17 August 1389) there was a great procession to mark the miracle wrought by our divine Lord with the blood of Saint Januarius. The blood, which is kept in a phial, turned into liquid just as if it had been in the living body of Januarius on that very day" (Sox, 1985; Rogo, 1982). The legend that the blood was collected by a serving woman from the stone on which Januarius was beheaded was apparently added around that time as well.
There are actually two phials of blood, one about two-thirds filled with dried blood, and the second containing only a few drops. The phials are permanently sealed in a glass case stored in a guarded vault, and the blood is exposed for veneration at three times during the year: on the Saturday preceding the first Sunday in May (the feast of Januarius's translation); on September 19 (the feast day that celebrates his martyrdom); and on December 16 (the feast day honoring him as patron saint of Naples). The ceremonies have been described as "boisterous," "unruly," and "hysterical," as crowds of people shout and invoke the saint to liquefy his blood (Rogo, 1982) although they currently take on a more solemn deportment. In May, the blood is taken in procession from the Duomo to the nearby church of Santa Chiara, and the liquefaction takes place there. In September, the liquefaction takes place on the altar of the Duomo. In December, the vault is unlocked and if the blood is found liquid or becomes liquid, it is shown to the people in the Cappella del Tesoro (Treasure Chapel) where the vault is. After the liquefaction takes place on the first day of the feast in September, the relic case is exhibited to the believers (who may kiss it) for 7 more days. During the night the blood is left on the altar (although others say it is locked in the vault), and the next morning it is again shown to the congregation. Some witnesses say that sometimes the substance solidifies overnight, in which case the populace must pray again, but not as long as the first time. The blood has been reported to remain liquid during the entire ceremony, while at other times it resolidifies before it is returned to the vault (Rogo, 1982). Very occasionally, it does not liquefy at all, to the confusion and anger of the Saint's devotees; according to one source, the last time this happened is said to have been when Naples elected a Communist mayor (Farmer, 1987). Sometimes the liquefaction takes place only after several days of prayer and this delay is also somehow seen as a bad omen. However, this belief is now almost a superstition, openly discouraged by the religious leaders themselves. As with most stories or legends of this nature, the truth is in the eye of the beholder!
The recent simulation of the phenomenon of the holy blood is not the first (Rogo, 1982). In the late nineteenth century, Professor A. Albini of the University of Naples was reported to have discovered that a solution of chocolate powder, sugar, casein, whey, salt, and water remained solid when left undisturbed but liquefied when shaken (Rogo, 1982; Albini, 1890). However, while his chocolate fudge did liquefy when shaken, it still remained very viscous. After a while, it separated into several layers and failed to solidify again. According to Rogo (1982), in 1906, Professor Guido Podrecci showed that calf's blood mixed with a chemical solution would liquefy when gently heated. This may be an error by that author (Rogo, 1982), since the calf's blood mixture was actually prepared by engineer Arnaldo Giaccio from Naples and the demonstration was then repeated in Rome in December of 1906. The demonstration, which was, according to Rogo (1982) rather unsuccessful, was organized by Guido Podrecca and Gabriele Galantara, editors of an anti-clerical socialist magazine named The Donkey or The Ass (L'Asino, 1906). There is little question that the recent work reported in Nature (Garlaschelli, 1991) is the most convincing. The procedure used to prepare the simulated blood is shown in Table I. The blood is a thixotropic gel of iron hydroxide, colloidal FeO(OH) of proper ionic strength.
Dissolve 25 g FeCl3 · 6H20 in 100 mL of water. Slowly add 10 g of CaCO3. Foaming and CO2 evolution will occur, so add slowly by stirring. A dark brown solution will be produced. Dialyze the solution against distilled water using cellophane tubing, parchment, or animal gut, changing the distilled water every 24 hours until it is no longer yellow. If using cellophane tubing, cut about one foot, wet it well and tie a knot at one end. If using parchment, attach to the end of a bottomless tube. Animal gut can be obtained from a butcher or also found in drugstores, sold as a prophylactic. After dialysis, the solution can be used as is or can be concentrated by gentle evaporation. Pour some of the solution into a small round flattened bottle, add a tiny amount of table salt (NaCl), and shake. Leave untouched and see if it has jelled. If not, add more salt. The setting qualities of the gel, such as how fast it sets or liquefies, can be adjusted by varying the solution concentration (by evaporation) or the amount of added salt. Thixotropics sols will equilibrate after one or more months, and they may gel incompletely and become only very viscous. The addition of a very tiny amount of salt should restore their original behavior. (Authors note: The procedure is necessarily abbreviated from the original directions and hints provided by Luigi Garlaschelli. To obtain a copy of that procedure, contact the author).
The validity of this replication of St. Januarius's miracle blood depends on establishing that the apparatus, chemicals and procedures used here were available in the fourteenth century. The balance, distillation (for preparing pure water), crystallization, evaporation, and filtration were all known before the third century A.D. (Szabadvary, 1966). Of course, a balance would not really be necessary if the mixture was prepared by trial and error, and rain water could be substituted for distilled water. Calcium carbonate (CaCO3) is one of the commonest minerals, being the main constituent of limestone. Sodium chloride (NaCl) or sea salt is also common and both natural minerals are described in ancient texts. Ferric chloride (FeCl3) exists as the mineral molysite, which is precipitated out of volcanic lava flows. It only occurs naturally in areas of volcanic activity, such as near Vesuvius in Italy (Garlaschelli, 1991).
From a historical standpoint, the weakest part of the replication procedure is the dialysis, which is used to purify the colloidal dispersion by removing the unreacted ferric chloride and calcium chloride byproducts of the reaction. The brown reaction product is stored on one side of a semi-permeable membrane (i.e., in a bag of cellophane, parchment, or animal gut) placed in distilled water. The dissolved salts then flow through the membrane into the distilled water until the concentrations on both sides are equalized. While pigments were stored in gut or bladder bags in the fourteenth century, the procedure as a mechanism for purification of colloidal dispersions was not established until the nineteenth century. However, Van Helmont showed experimentally that salt can pass with water through a bladder in the early part of the seventeenth century (Partington, 1961), and it is possible that an earlier researcher accidentally stumbled upon this phenomenon. It is only a small step from filtration to dialysis. The great Greek physician, Hippocrates had great interest in the purification of water (Szabadvary,1966) and precipitated lakes produced for artistic purposes were filtered using the so-called Hippocrates' Sleeve, a filter manufactured with a closed tube of felt, not unlike a tube of parchment (Cenni, 1971). While a thixotropic gel can be obtained without dialysis by mixing ferric chloride, calcium carbonate, and water in exact proportions (Guthknecht, 1946), the simulated blood prepared in this manner has been found to exhibit thixotropic properties for only a short period of time.
Since the only historically questionable step is the dialysis, we experimented with procedures to eliminate it. The procedure described in Table 1 for the preparation of the simulated blood was followed up to the point of dialysis. Then, rather than using dialysis, we allowed the brown solution to sit undisturbed for 24 hours. At this point it had formed a thick gelatinous layer on top of the bulk of the solution. The layer was removed , a few drops of distilled water were added, and the resulting mixture was ground with a glass mortar and pestle to break up clumps and hasten evaporation until it would solidify upon sitting undisturbed for several minutes. The mixture was then placed in two small glass vials. The remainder of the bulk solution from which the gelatinous layer was removed solidified after another 24 hours and was treated similarly. Also, to generate a more "blood red" color than the "yellow-brown" of the original gel, we added a few grains of potassium thiocyanate to each vial. The reaction of thiocyanate with iron is often used in "chemical magic" to produce a simulated blood color. While "blood acid" was only first mentioned in the eighteenth century by Winterl (Szabadvary, 1966), thiocyanate can be prepared by fusing a cyanide-containing compound and sulfur. The recognition of cyanide as a poison in plant materials such as bitter almonds, cherry laurel leaves or peach pits goes back to the ancient Egyptians, who spoke of "the penalty of the peach" (Sykes, 1981). The addition of thiocyanate to a dialyzed solution of the gel will not work, since there are no free ferric ions to form the colored complex. However, other colored species could certainly be added to the mixture to produce the appropriate color. The holy blood of Saint Januarius has been described as "a solid black congealed mass" (Sox, 1985) or "dark brown" (Rogo, 1982) when dry, and it transforms during liquefaction to "a red liquid" (Sox, 1985) or "lighter...then turns yellowish red and finally scarlet" (Rogo, 1982). This is similar to the behavior of the simulated blood produced by this procedure, which solidifies when motionless for about a minute and rapidly liquefies when shaken.
After several days the two vials of blood prepared from the bulk, undialyzed solution failed to coagulate, forming a flocculant precipitate of iron hydroxide which settled to the bottom of the container. The vials prepared from the gelatinous layer above the bulk, undialyzed solution coagulated for approximately three months, and then finally failed. Also, after approximately six weeks they required regeneration by the addition of a small amount of salt and evaporation. They were at this point much less solid in appearance when coagulated. It is possible that some purification takes place during the formation of the layer on top of the bulk solution, in that impurities are more soluble in the bulk liquid , similar to the purification that occurs during a crystallization process, producing a more stable thixotropic gel. The thiocyanate probably plays no more than a cosmetic role and may be unnecessary, although some stabilization of the gel might be due to complexation of free ferric ion. Certainly, dialysis is the more straightforward although historically uncertain answer. There also may be environmental factors involved such as the frequency and magnitude of agitation and temperature. The simulated blood that we prepared in this experiment was shaken several times a day and certainly not venerated as a relic.
Beyond the chemistry in the preparation of simulated blood, the question of motivation arises. It is not well known that Albertus Magnus (1193-1280) and his student, Thomas Aquinas (1226-1274), both canonized as saints and recognized as among the greatest intellects in the history of the church (Butler, 1955), were also qualified alchemists (Szabadvary, 1966; Sadoul, 1972). Albert the Great was a bishop, a professor at the Paris University and one of the outstanding scientists of the Middle Ages. In spite of the fact that many alchemists were priests, Pope John XXII forbid the study of alchemy in a Bull in 1317: "Alchemists deceive us and promise what they cannot perform ... if any members of the clergy are found among alchemists, they will receive no mercy ..." It is little wonder that alchemists kept their work secret. And the clerical nature of alchemical study is also not surprising, since the Abbeys were among the only places where learning could be cultivated in peace (Sadoul, 1972).
Today, a large percentage of the world's population believes that through transubstantiation, bread and wine physically change into the body and blood of the Son of God. Is it not possible that 650 years ago a Neapolitan cleric/alchemist, who might regularly pray to his patron saint, Januarius, accidentally discovered the thixotropic properties of the mixture of molysite and limestone? Might he not believe that the material had taken on the form of the blood of his patron saint? Better to present his discovery as the finding of Januarius's blood and receive acclaim, then present it as the result of an alchemical procedure and receive "no mercy" from Pope John XXII! Furthermore, in 1389, the Duomo of Naples was being built up and many artists from all over Italy were present. The king was then Robert of Anjou, described as an extremely religious person, and a "holy blood relic" was certain to please him.
Several unique events or claims have been associated with the phenomenon of Saint Januarius's blood. Let's study some of them:
(a) "Failure of the blood to liquefy is considered a bad omen. It did not liquefy in May 1976, just before the worst earthquake in Italian history struck" (Rogo, 1982). Another reference (Sox, 1985) relates that "in May 1976 the liquefaction did not occur and nothing unusual happened." While an earthquake did occur in Italy in 1976, it can hardly be called "the worst earthquake in Italian history." The Great Italian Earthquake occurred on November 23, 1980, killing 3,000 people and leaving 300,000 homeless (Walker, 1987). Italy is seismically active, and earthquakes happen there quite often.
(b) "The substance stored in the phials is definitely blood. Several scientists at the University of Naples examined the phials in 1902. By shining a beam of light through the glass case they were able to make a spectroscopic analysis of the relics. This analysis verified that the phials contain blood, though it is possible that it has been contaminated by a foreign substance" (Rogo, 1982). In 1989, further examination was made of the blood using spectroscopic analysis similar to that used in 1902, but with photographic detection (Bollone, 1989) which claimed to confirm the 1902 results. The instrumental difficulties in making an absorbance measurement in a phial within a glass case using equipment available in 1902 are immense. The measurements had to be made by visual estimation of light levels, since photographic and photoelectric detection methods weren't used until 1910 (Laitinen, 1977). Since the liquid or solid blood is too dense to permit an absorbance measurement, the absorbance spectrum of the film of blood remaining on the wall of the phial would have to be measured. This would be very difficult, particularly if the substance were really liquid blood, because the film thickness would not be stable over an extended time (not to mention the inhomogeneity of the four intervening glass surfaces, two from the ampoules and two from the relic case). In any event, since the material in the phial "looks like blood," it must have a similar absorbance spectrum, and any such test could not possibly be used to determine that the material "is definitely blood." unless high resolution spectroscopic instrumentation with an intense radiation source, capable of accurate wavelength identification and resolution of narrow absorption bands was available to make the measurements.
Scientists in 1902 could only visually measure absorbance spectra down to a wavelength of about 400 nm, since the sensitivity of the human eye rapidly decreases at shorter wavelengths. This was verified from spectra of blood published around that time (Mathews, 1916) and by repeating the visual measurement experiment using a hand-held spectroscope. Bollone (1989) reported that a candle flame was used in the 1902 experiment as the light source and that wavelength calibration was obtained by introducing sodium chloride into the candle flame. The original source of information for the 1902 spectroscopy is a book by Sperindeo (1903). Bollone reproduced the experiment of 1902 using a prism spectroscope, except that a stronger radiation source and photographic detection was used. Although not specifically mentioned by Rogo (1982) or Bollone (1989), it could be assumed that the conclusion that the substance contained blood was based on the observation of an absorption band in the yellow region of the visible spectrum. Hemoglobin exhibits such a band, as do many other substances including components of glass. Such a band does appear in one of the spectra taken by Bollone (Figure 9, p. 74) but is very difficult to observe in the other figures (10 through 12) shown at lower magnification. The spectra shown by Bollone (1989) show no wavelength calibration, and exhibit some unusual anomalies, such as extremely sharp color divisions and distortions in the spectral distributions. The conclusion that the reliquary contained blood was very far from scientifically valid.
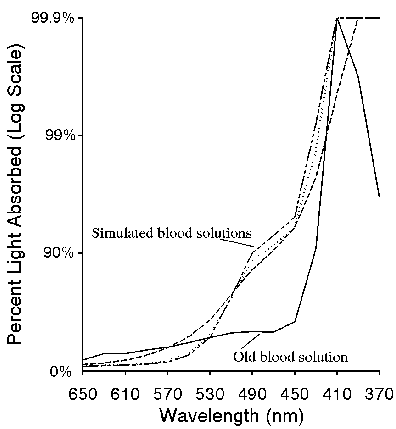
The validity of any simulation of the miracle of Saint Januarius requires that, at the very least, a spectroscopic study be carried out on the media used to simulate the holy blood. Using a more modern absorption spectrophotometer and photoelectronic detection, we compared the absorbance spectrum of old blood to three versions of the FeO(OH) simulated blood. The result is shown in figures 1 and 2. In figure 1, samples of the old blood, (approximately 10 years old, stabilized with heparin), and the simulated blood (with and without dialysis, and without dialysis but with thiocyanate) were spread as a film on one surface of a 1 cm3 quartz cuvette and the absorbance spectrum measured in the visible (650-370 nm) region of the electromagnetic spectrum. While the simulated blood films were stable and absorbances were reproducible over the 15 min measurement time, the real blood film was stable for less than a minute. Absorbance of that film had to be measured very rapidly. Water was then added to the cells to provide a homogeneous absorber and the absorbance measurements were repeated, as shown in figure 2. Figure 3 shows a wavelength scan of fresh blood, old blood, and the simulated blood.
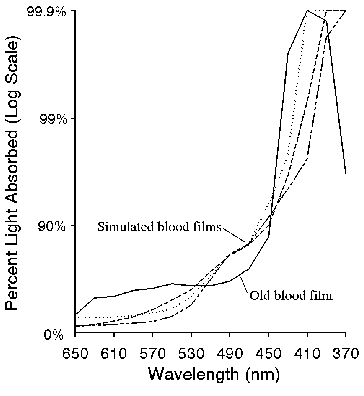
Figure 1. Absorbance of real (solid line) and simulated (dashed lines) blood films.

Figure 2. Absorbance of real (solid line) and simulated (dashed lines) blood solutions.
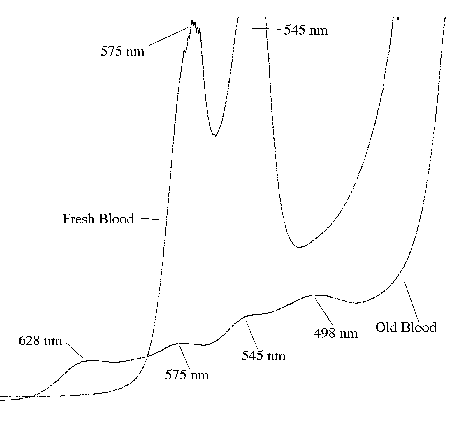
Figure 3. Absorbance of fresh blood and old blood taken with a rapid-scanning absorption spectrophotometer.
The recent measurements by Bollone (1989) can also be strongly called into question. Measurements were made on similar old glasses to rule out deformations or anomalous absorption bands. The correct method would have been to make measurements at two different film thicknesses and determine the spectrum of the reliquary contents by difference. In fact, several glasses containing inorganic ions exhibit absorption bands in the region of interest. Cobalt glass (Weyl, 1951) , known since antiquity, as well as rare-earth glasses could be responsible for the observed absorption band. Even small quantities of impurities in the glass (which is, of course, likely inhomogeneous) could generate an absorption band specific to the glass in the reliquary. Perhaps the most interesting statement is that made by Rogo (1982) in quoting the 1902 spectroscopic work: "the phials contain blood, though it is possible that it has been contaminated by a foreign substance.". Perhaps a better statement would be "that the phials contain a foreign substance, perhaps contaminated by blood". The appearance of an absorption band in the yellow region of the spectrum is extremely weak evidence, particularly because of the lack of wavelength identification and the spectral anomalies that are clearly evident.
(c) "A number of other cases of 'liquefying blood' miracles have been documented ... the great majority of liquefying blood miracles occur in the south of Italy, in or around Naples. This geographical quirk, and the fact that none of these miracles predate the miracle of St. Januarius, may provide a clue to the nature of the phenomenon. Could it be that the news of St. Januarius's miracle in Naples which spread throughout southern Italy sometime in the fourteenth century caused congregations in other churches that housed blood relics to pray for or expect similar wonders (Rogo, 1982)?" Could it be instead that the chemical knowledge to make liquefying Holy Blood was spread around southern Italy in the fourteenth century?
(d) The phase transition is due to temperature changes and not to any thixotropic characteristics of the substance. Although temperature may certainly play a part in thixotropic characteristics, the temperature effect has been rejected by many (Rogo, 1982) based on evidence that the reliquary was cool when kissed and that the liquefaction was independent of the temperature in the cathedral.
(e) The color, volume, and weight of the contents of the reliquary vary during liquefaction. This is mostly anecdotal evidence. No records are available about changes in color and volume but the story is often repeated. Weight variations were recorded in 1902 and 1904 (up to 28 g on an estimated amount of "blood" of 30 mL, but 3% on the total weight of the relic case). No type of balance was reported (La Civilta' Cattolica, 1905). Moscarella (1989) writes that "Tests performed during the last five years by using electronic balances failed to confirm any weight variation. Also, volume variations seem no longer to be reported."
(f) The blood is genuine and is liquefied by the prayers of the crowd. There has been speculation (Rogo, 1982) that the behavior of the holy blood results from psychokinetic influence of the crowd venerating the relic. This cannot be ruled out by the present study, since such an effect might also impact a chemical reaction. After all, the relic has lasted 600 years. However, it has been reported that another miracle blood failed in the eighteenth century (Rogo, 1982). If crowd emotion were the influencing factor, the miracle blood would not have failed. Why would a saint suddenly decide no longer to liquefy his blood?
There have been many claims concerning the blood of Saint Januarius. The evidence is very strong that the blood of this saint, as well as other liquefying miracle blood, is actually a thixotropic gel. While the exact composition of these relics is still unknown, the simulation demonstrated in this article is historically feasible in terms of motivation and alchemical knowledge and the results of previous studies can be explained. The behavior of the simulated blood correlates well with the characteristics of the actual relic. Certainly, further work is needed to study the stability of different concentrations as well as stabilizers used in medieval times. However, until scientific analysis proves otherwise, the most logical assumption is that the chemical knowledge to make liquefying holy blood was spread around Italy in the fourteenth century and that it is a thixotropic gel, perhaps mixed with contaminants that simulate the color and spectrum of blood. Since we know what to look for, measurements with modern spectrophotometric equipment using not only molecular absorption but fluorescence spectrometry and raman scattering (Baeyens, 1991) should provide the answer. It remains in the domain of the Church to allow the measurements to be made by highly-qualified spectroscopists with modern equipment, while still respecting the sacred nature of the relic.
One author (MSE) gratefully acknowledges Walter Rowe for forensic guidance and the editorial assistance of Lys Ann Shore, Editor of the National Capital Area Skeptical Eye, as well as the many helpful suggestions of colleagues. The other author (LG) gratefully acknowledges his colleagues Franco Ramaccini and Sergio Della Salla who coauthored the original paper in Nature (see references). Reprint requests may be directed to either author.
![]() Return to the
Januarius Blood page
Return to the
Januarius Blood page
Page prepared by: Mike Epstein
Last Modified: 30 April 1999