The Allison
Magneto-Optic Method of Chemical Analysis provides a unique opportunity
for classroom discussion on a number of topics. It did not start
off as pathological science, since the effect could have been
a real physical phenomenon based on the state of scientific knowledge
at the time of the investigation. It degenerated into pathological
science because of poor experimental design and experimenter bias.
A full understanding of the concepts involved in the experiment
on the part of students is quite an accomplishment, since it requires
an understanding of polarized radiation, electrical current, magnetic
fields, and spectroscopic sources and detectors. This is one of
the most useful cases of pathological science for educational
purposes.
During the first 40 years of the 20th
century, scientists were eagerly trying to find real evidence
for the existence of elements 85 and 87, which had been predicted
by Mendeleyev in the late 19th century. It was
during this time that Fred Allison, an American physicist, devised
an analytical method that he called the magneto-optic method
of chemical analysis.
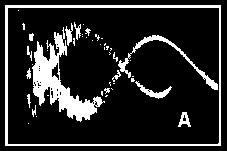
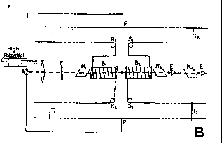
Figure 1. The Magneto-Optic Effect
(A) Spark waveform (B) Schematic diagram of the apparatus
The method was based on the comparison
of the difference in response of the Faraday effect induced in
different liquids (Allison, F. (1927). The effect of wavelength
on the differences in the lags of the faraday effect behind the
magnetic field for various liquids. Physical Review,
30, 66-70). The Faraday effect involves the rotation
of polarized light passing through a liquid, and induced by a
magnetic field applied to the liquid. He constructed an
apparatus in which light from a high-voltage spark was directed
through crossed polarizers and two tubes containing the liquids
to be analyzed, and surrounded by coils of opposite winding.
The time at which the magnetic field was applied to the different
liquids could be varied by adjusting the distance the electric
current had to travel to reach the cells. The observer looked
for a minimum in the light from the spark, which indicated the
delay in the appearance of the Faraday effect (relative to the
reference liquid) after the magnetic field had been applied.
Allison first applied this procedure to simple
solutions of carbon bisulfide and hydrochloric acid and later
to mixtures. He became convinced that compounds would retain
their individual maxima in a mixture, regardless of the other
components. He claimed a sensitivity down to less than part-per-billion
levels, and he began to use his method to search for the elusive
elements 85 and 87. He rapidly found them and published
studies on spectra and compounds of virginium and alabamine.
His results were replicated in part by several other laboratories,
but detailed examination by others (MacPherson, H. G. (1934).
An investigation of the Magneto-Optic Method of Chemical Analysis.
Physical Review, 47, 310-315) proved conclusively
that the maxima observed by Allison and others "had no objective
reality" and "were not a function of the chemical solutions
used." The Allison magneto-optic method disappeared
from the pages of scientific journals. In retrospect, the
modern analytical spectroscopist can easily find errors in Allison's
reasoning. He never offered a substantial theory to explain
his observations, but more importantly, he ignored several experimental
factors that limited his approach from the start. This was
a classic example of the investigator finding what he believed
in. But it certainly didn't hurt his career, since his biography
lists him as head of the Auburn physics department and dean of
the graduate school in 1953, with the physics building at Auburn
named in his honor. Furthermore, he is still listed as the
discover of astatine (i.e., element 85, alabamine) in the 1991
Concise Columbia Encyclopedia and the work at Alabama Polytechnic
(later Auburn University) is similarly credited in the 1994 and
1996 editions of Microsoft Encarta.
The so-called Allison Effect was one of the
instances of pathological science discussed by Irving Langmuir
in his 1953 lecture that was reprinted in PHYSICS TODAY, Oct 1989,
P. 36. Why did Allison make the assumptions that led him to the
claims that he made?
Modern-day spectroscopists can perhaps judge
Allison far less harshly than Langmuir. Let's examine his
apparatus in detail. Look at the instrument diagram. This
scheme might have worked if the radiation from the spark light
source was temporally very short (ns) and very stable, which it
was not! Unfortunately, a single light pulse from a spark
(which is repeated rapidly as the capacitor charges and discharges)
is on the order of microseconds, but this was not general knowledge
at the time. Anyway, the idea was to take the light pulse
from the spark and pass it through a polarizing prism. The
light pulse then passes through two cells that are wrapped with
wire. The wire goes to the spark circuit so (theoretically)
when the capacitor discharges across the spark gap, current flows
through the coils and a magnetic field is applied to the cells.
With certain liquids in the cell, the plane of polarization of
the light passing through them is rotated. Now the coil
around the second cell is wound completely opposite to the first
cell. Therefore, if the same liquid is in each cell, and
the magnetic field is applied to each cell at the same time, the
second cell will reverse the Faraday effect on the first cell,
thus restoring the direction of polarization defined by the first
polarizer. A final polarizer (Nicol prism) is placed after
the second cell and oriented 90 degrees out of phase of the first
polarizer. Thus, no light should pass through the system.
Now, say we have two different liquids in the cells and there
is a delay between the application of the magnetic field to the
cell and the appearance of the Faraday effect, and this delay
depends on the identity of the liquid. This is what Allison
was claiming. If the light pulse passes through the first
cell (containing liquid A) and its polarization is changed, it
will pass through the second cell (containing liquid B with a
longer delay in the Faraday effect) before the Faraday effect
in that cell can restore the direction of polarization defined
by the first polarizer. Therefore, light will pass the system.
Now, all we have to do is shorten the length of the wire to the
second cell (a distance corresponding to the time delay of the
Faraday effect in the second liquid) until we see a minimum in
the light throughput of the system (i.e., the Faraday effects
of both liquids overlap) and we have defined the time delay, which
is characteristic of the liquid B.
He started this work with J. Beams at the University
of Virginia and Beams himself continued his work with this effect
and sparks but then went on to other things. It is not clear
whether he ever discovered his mistake, but he certainly never
pushed the technique. On the other hand, Allison began to
make grandiose claims that compounds when dissolved gave delay
minima corresponding to the compounds (NOT the ions!) and that
the effect was the same down to 10-8 M and then disappeared.
By the early 1930's, the ACS eventually refused to accept any
more papers on Magneto-optic Spectroscopy, but not before a good
deal of debunking took place and Allison claimed to pass *blind*
tests of his methodology.
What is most telling is that the "hero"
of the N-Rays affair, Robert Wood, was taken in by the Allison
Effect. It is referenced in a very straightforward manner in his
book, Physical Optics! Why was Wood fooled? Perhaps
because at the state-of-the-art in knowledge of spark spectroscopy
in the 1920s, Allison's technique might have worked. It
was Allison's dogmatic refusal to accept that he made a mistake
that made this episode truly pathological.
And, as mentioned previously, it looks like we are
still being fooled by the Allison Effect ... at least Columbia
University Press and Funk & Wagnalls are...
Contributors:
[Thanks are due to Walter Rowe, Professor of Forensic Science
at George Washington University, Alex Scheeline, Professor
of Chemistry at the University of Illinois-Urbana]
Page prepared by: Mike Epstein
Last Modified: 30 April 1999